Why enzyms play a curcial role in biosensors
Biosensors are powerful analytical devices that detect and measure specific analytes, revolutionizing fields like healthcare and environmental monitoring. Since the first commercial biosensor was introduced in the 1970s, these devices have saved millions of lives (Clark et al., 1962). However, the enzymes that are essential to biosensors have experienced limited advancements over the past few decades, with most progress concentrated on enzymatic amperometric sensor technology.
DirectSens, founded in 2013, is dedicated to advancing biosensor technology by engineering improved enzymes, inspired by nature. Enzymes are crucial for determining a biosensor’s specificity, stability, and overall performance. Most biosensors utilize oxidase or dehydrogenase enzymes in combination with a two-step detection mechanism to generate a measurable signal (Guilbault & Lubrano, 1973; Updike & Hicks, 1967).
DirectSens, founded in 2013, is dedicated to advancing biosensor technology by engineering improved enzymes, inspired by nature. Enzymes are crucial for determining a biosensor’s specificity, stability, and overall performance. Most biosensors utilize oxidase or dehydrogenase enzymes in combination with a two-step detection mechanism to generate a measurable signal (Guilbault & Lubrano, 1973; Updike & Hicks, 1967).

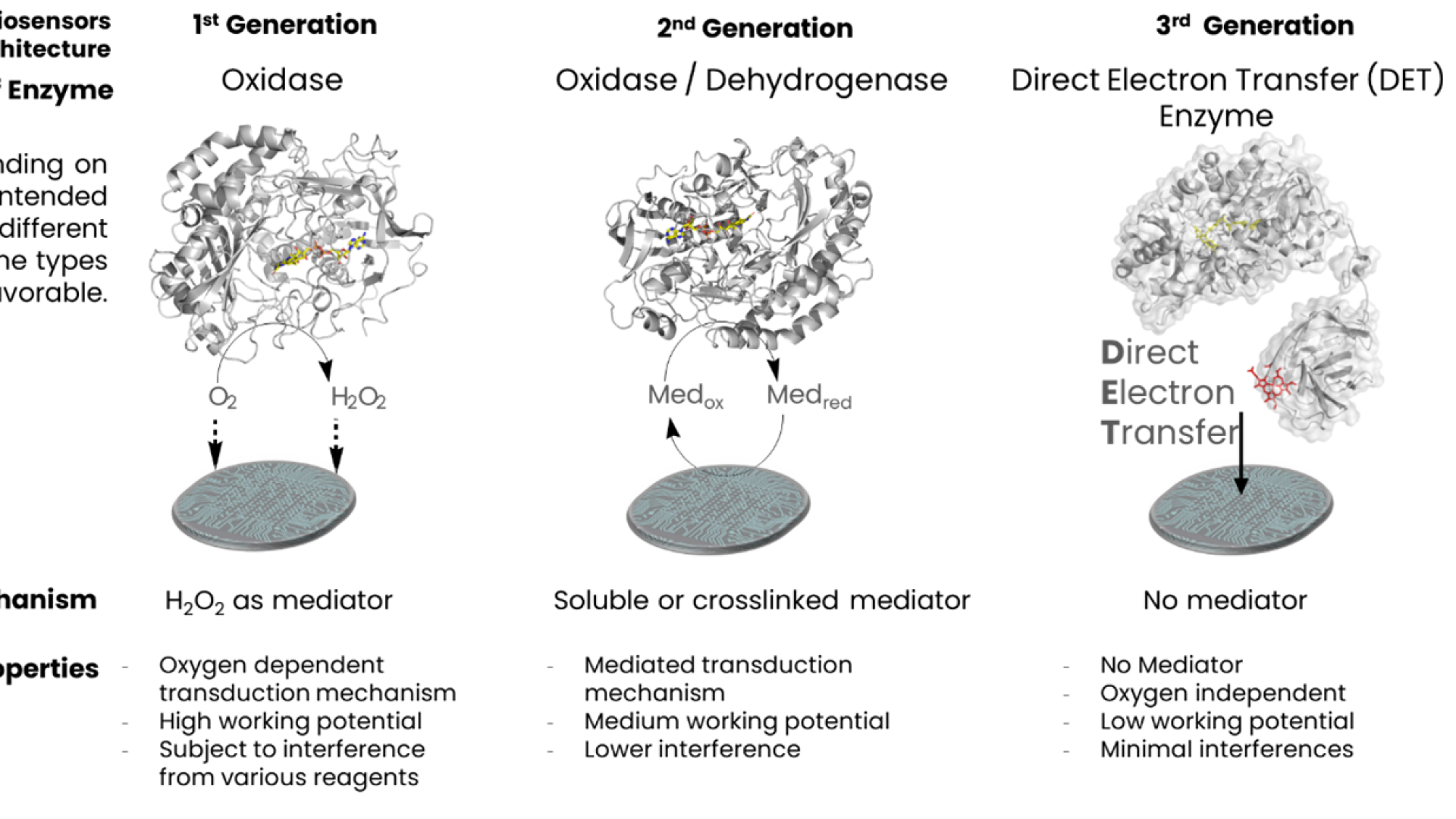
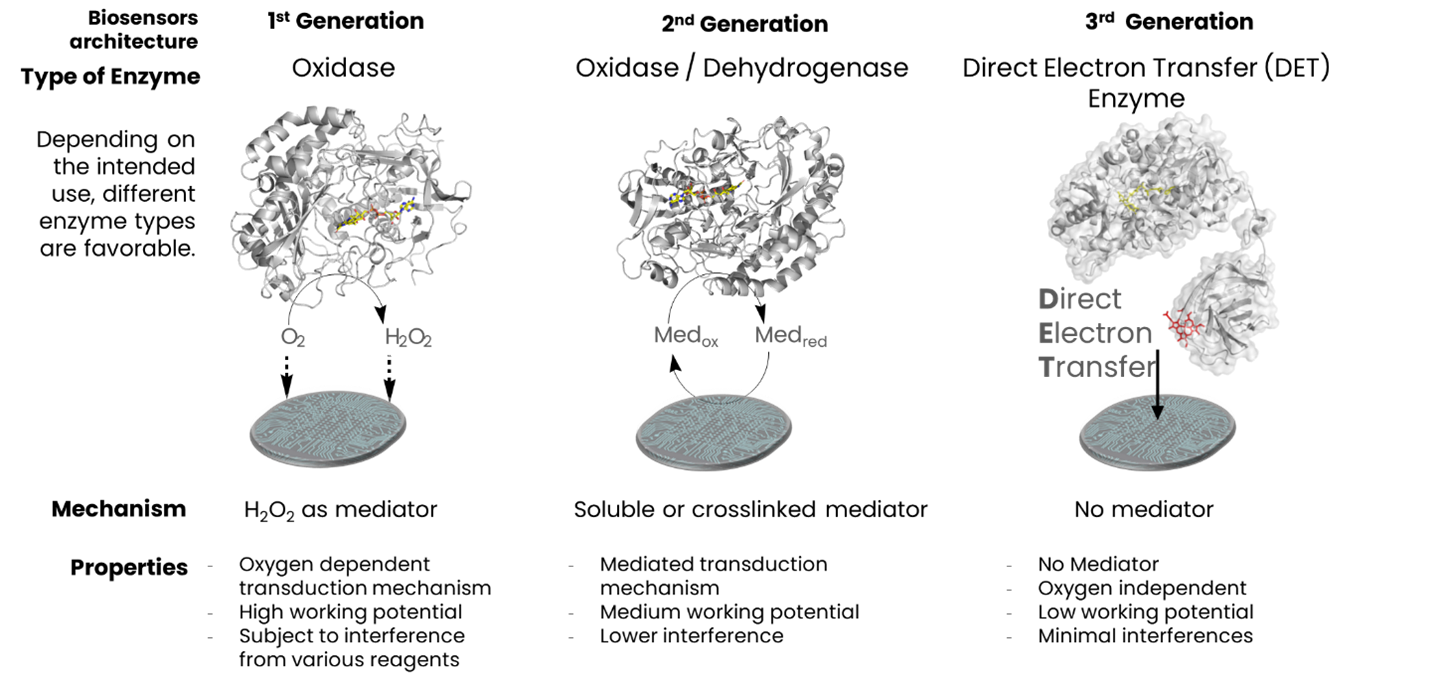
Three generations of enzymatic biosensors have been developed, each relying on redox enzymes coupled with electrodes: First-generation biosensors detect electrochemical signals from oxygen or hydrogen peroxide produced by the enzyme-catalyzed reaction. However, these sensors are oxygen-dependent and susceptible to interference due to the high working potentials required (Clark & Lyons, 1962). Second-generation biosensors incorporate redox mediators to facilitate electron transfer between the enzyme active site and electrode. Some of these enzymes, like flavin mononucleotide (FMN)-dependent enzymes, work at medium to low potentials and are oxygen-independent (Guilbault & Montalvo, 1969). Third-generation biosensors utilize enzymes capable of direct electron transfer (DET) from the redox center to the electrode surface, eliminating the need for mediators. These sensors operate at low potentials and are both oxygen and mediator-independent (Ghindilis et al).
DirectSens has pioneered the commercialization of third-generation biosensors by developing enzymes with DET capabilities. This represents a significant advancement in biosensor technology, enabling more reliable, sensitive, and specific analyte detection.
References
Clark, L. C., Lyons, C. (1962). Electrode systems for continuous monitoring in cardiovascular surgery. Annals of the New York Academy of Sciences, 102(1), 29-45.
Clark, L. C., Noyes, L. K., Spokane, R. B., Hartdegen, F. J., & Weiss, G. H. (1978). U.S. Patent No. 4,073,713. Washington, DC: U.S. Patent and Trademark Office.
Guilbault, G. G., Montalvo, J. G. (1969). A urea-specific enzyme electrode. Journal of the American Chemical Society, 91(8), 2164-2165.
Guilbault, G. G., Lubrano, G. J. (1973). An enzyme electrode for the amperometric determination of glucose. Analytica Chimica Acta, 64(3), 439-455.